Translational Science Benefits
Summary
Cancer is a global pandemic, increasingly burdening individuals, families, and healthcare systems worldwide.1 Brain tumors are the fifth most common type of cancer in the United States.2,3 Diagnosing and treating brain cancers is complicated by the blood-brain barrier (BBB). The BBB is a physical barrier that prevents the circulation of genetic information such as disease-specific biomarkers from the brain to the circulatory system. Current methods for brain cancer diagnosis include neuroimaging, surgical resections, tissue biopsies, and lumbar punctures.1 These methods are invasive, risky, and can be uncomfortable. A promising, noninvasive new method to bypass the BBB is sonobiopsy. Sonobiopsy combines transcranial low-intensity focused ultrasound with intravenously injected microbubbles to open the BBB. By using this inexpensive and rapid method, physicians can obtain blood samples with adequate tumor biomarkers to determine the brain cancer type and stage and assess whether a treatment is effective.
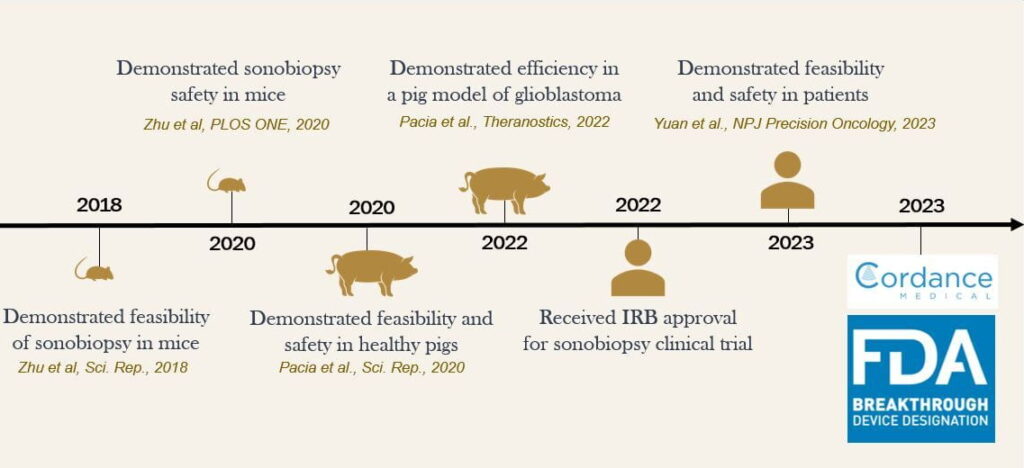
Hong Chen, PhD, Eric Leuthardt, MD, and their collaborators set out to test the feasibility of sonobiopsy to measure biomarkers released by brain tumors. The researchers initially tested the method in mice. The group of mice treated with the sonobiopsy had detectable levels of circulating tumor RNA and DNA markers in their blood, indicating that the method opened the BBB and allowed for the biomarkers to cross the barrier. Following this study in mice, the sonobiopsy method was tested in pigs, and it was deemed to be both safe and efficient. Sonobiopsy was validated in human patients,4 and it is now being trialed on a larger scale in human patients with glioblastoma.7 The larger trial will help determine the safety and efficacy of the method for humans.
Significance
Because sonobiopsy is noninvasive and nondestructive to brain and other tissue, this method has essentially created a new field of study for the brain.5 The genetic and molecular information made available by sonobiopsy has the potential to drastically change brain cancer diagnosis and treatment. The method could be extended to other conditions too, like developmental disorders, psychiatric illnesses, and neurodegenerative diseases. This method is also clinically significant as it removes a barrier to allow existing oncology liquid biopsies to be used.5
Dr. Chen and Dr. Leuthardt patented and licensed the sonobiopsy work to Cordance Medical to manufacture the NeuroAccessTM device. This device performs sonobiopsies at the patient’s bedside and was granted “Breakthrough Device Designation” by the FDA.5 Such a designation accelerates the device’s development to expedite patient access.6,7
Traditionally, certain medicines have been unable to cross the BBB, thus limiting available treatments for the brain. Both Cordance Medical and Dr. Chen’s lab are exploring using this method to help deliver medicines and to conduct liquid biopsies for other conditions such as Alzheimer’s disease, Parkinson’s disease, brain metastases, and other neurological diseases.5 Dr. Chen noted that “we can now start to interrogate diseases that traditionally do not undergo surgical biopsies, such as neurodevelopmental, neurodegenerative, and psychiatric disorders.” 5

The portability of the NeuroAccessTM device and its ease of integration into established clinical workflows means it can be used at the patient’s bedside, thus requiring no additional specialized equipment.
Benefits
Demonstrated benefits are those that have been observed and are verifiable.
Potential benefits are those logically expected with moderate to high confidence.
The sonobiopsy method was developed in 2018 using animal models. demonstrated.
Clinical
The sonobiopsy method was validated in humans in 2023 using high-grade glioma patients. demonstrated.
Clinical
Cordance Medical used the licensed sonobiopsy technology to create the NeuroAccess device.11 demonstrated.
Clinical
Clinical trials testing sonobiopsy for safety and efficacy in glioblastoma patients began in April 2022.8-10 demonstrated.
Clinical
Sonobiopsy has the potential to perform biopsies for other conditions such as Alzheimer’s disease, Parkinson’s disease, brain metastases, and other neurological diseases. potential.
Clinical
Sonobiopsy may aid drug delivery by providing monitoring of drug effects. potential.
Clinical
The sonobiopsy technique increases accessibility to health care as it does not require invasive and destructive methods (surgical biopsies) or a high-tech scanner to obtain a blood sample that reflects the gene expression of brain lesions. demonstrated.
Community
The sonobiopsy technique can enhance health care delivery as sonobiopsies offer potential for analysis for hard-to-reach brain tumors. potential.
Community
The sonobiopsy technique can enhance life expectancy and quality of life for brain cancer patients as it provides a noninvasive molecular diagnosis. potential.
Community
The sonobiopsy patent has been licensed to Cordance Medical. demonstrated.
Economic
Dr. Chen and Dr. Leuthardt are listed as inventors for the sonobiopsy patent. demonstrated.
Economic
This research has clinical, community, and economic implications. The framework for these implications was derived from the Translational Science Benefits Model created by the Institute of Clinical & Translational Sciences at Washington University in St. Louis.12
Clinical
The sonobiopsy technique allows for a more accurate diagnosis of brain cancer than traditional diagnostic practices. It is particularly advantageous for cancers where tumors are located at inoperable brain sites or occur across multiple brain regions.13 This method offers precise targeting of various tumor areas, resulting in spatially localized release of biomarkers for enhanced diagnosis.
It is a noninvasive procedure.13 “It’s like doing a brain biopsy without the dangers of brain surgery,” said Dr. Leuthardt.5 This means that sonobiospy can decrease the burden for both the patient and the physician. Further, sonobiopsy can be performed at the patient’s bedside. Until now, researchers have utilized a costly ultrasound device combined with an MRI scanner, restricting its use to locations with MRI capabilities. Dr. Chen’s team developed a portable, handheld ultrasound probe integrated with a stereotactic pointer. This integration seamlessly fits into the clinical workflow without requiring extra training for neurosurgeons.14
The NeuroAccessTM device is portable, noninvasive, and painless.15 It is designed for use in a community clinic, and it should require an outpatient visit of no more than 30 minutes. The device can facilitate liquid biopsies (SonoBiopsy) as well as drug delivery (SonoScript). Both methods are being tested in brain tumor patients, Alzheimer’s patients, Parkinson’s patients, and depression patients.

Community
Current brain cancer diagnosis relies on risky, expensive, and potentially uncomfortable methods, like MRIs, CT scans, surgical resections, and lumbar punctures. These methods are highly specialized and costly. The NeuroAccessTM device increases accessibility to diagnostic care for brain cancers as it is noninvasive and therefore more acceptable for patients. The genetic information made available from the sonobiopsy technique in combination with imaging information gives clinicians more information to make data-driven treatment decisions, especially for patients who are poor surgical candidates.4 Sonobiopsy’s accessibility helps facilitate multiple samples taken over time to monitor tumor recurrence and treatment response. These steps lead to increased quality of health care for brain cancer patients and potentially increased quality of life due to more effective care. Thus, this type of work is incredibly important for potentially increasing life expectancy for glioblastoma and all brain cancer patients. Research made possible by sonobiopsy may lead to new and effective therapies for brain tumors.
Economic
Dr. Chen and Dr. Leuthardt are both inventors of the noninvasive and localized brain liquid biopsy using focused ultrasound technology.11 This technology is patented (US Patent. US11667975B2), has been licensed to Cordance Medical, and provides the basis for the NeuroAccessTM device.7 Dr. Chen participated in the Equalize 2021 competition, where she gave a pitch for sonobiopsy and outlined its current and potential future market sizes.
Lessons Learned
In the trial of human patients, the sonobiopsy technique did not cause tissue damage or induce brain inflammation, which is an important finding for patient safety. The sonobiopsy technique also enriched patient-specific tumor DNA variants in the blood circulation.
- Connal S, Cameron JM, Sala A, et al. Liquid biopsies: the future of cancer early detection. J Transl Med. 2023;21:118.
- New York State Department of Health. About brain cancer. Published April 2016. Accessed March 22, 2024.
- Neff C, Price M, Cioffi G, et al. Complete prevalence of primary malignant and nonmalignant brain tumors in comparison to other cancers in the United States. Cancer. 2023;129(16):2514-2521.
- Yuan J, Xu L, Chien CY, et al. First-in-human prospective trial of sonobiopsy in high-grade glioma patients using neuronavigation-guided focused ultrasound. Npj Precis Oncol. 2023;7(1):1-11.
- Focused Ultrasound Foundation. Clinical Trial Results: Noninvasive Brain Tumor Biopsy. Clinical Trial Results: Noninvasive Brain Tumor Biopsy. Published October 12, 2023. Accessed March 22, 2024.
- Jonsson A. Cordance Medical’s NeuroAccess Receives FDA Breakthrough Device Designation for Liquid Biopsy in Brain Tumors » Cordance Medical. Cordance Medical. Published October 25, 2023. Accessed April 25, 2024.
- Sauerwin K. Device for noninvasive brain biopsies via blood draw moves closer to market approval – The Source – Washington University in St. Louis. The Source. Published November 15, 2023. Accessed April 25, 2024.
- National Library of Medicine. Study Details | Sonobiopsy for Noninvasive and Sensitive Detection of Glioblastoma . ClinicalTrials.gov. Published December 8, 2023. Accessed April 4, 2024.
- Miller B. Focused ultrasound technique gets quality assurance protocol. The Source. Published March 27, 2024. Accessed April 25, 2024.
- Chien CY, Xu L, Yuan J, et al. Quality assurance for focused ultrasound-induced blood-brain barrier opening procedure using passive acoustic detection. eBioMedicine. 2024;102:105066.
- Leuthardt E, Dunn G, Chen H, Petti A. Methods and Systems for noninvasive and localized brain liquid biopsy using focused ultrasound.
- Luke DA, Sarli CC, Suiter AM, et al. The Translational Science Benefits Model: A New Framework for Assessing the Health and Societal Benefits of Clinical and Translational Sciences. Clin Transl Sci. 2018;11(1):77-84.
- Zhu L, Cheng G, Ye D, et al. Focused Ultrasound-enabled Brain Tumor Liquid Biopsy. Sci Rep. 2018;8(1):6553.
- Bhandari T. Noninvasive, ultrasound-based brain biopsy is feasible, safe in people. Washington University School of Medicine in St. Louis. Published September 27, 2023. Accessed March 22, 2024.
- Cordance Medical. Our NeuroAccess Device can enable a liquid biopsy and improve drug delivery to the brain. Cordance Medical. Accessed March 22, 2024.