Translational Science Benefits
Summary
Premature infants are at higher risk for brain injuries that can cause lifelong impairments, such as cerebral palsy. This research is developing the first treatment to reverse the damage of these brain injuries, using a molecule found in human breast milk.
The goal of this work is to develop an intravenous formulation of an oxysterol (TT-20), which was found in human breast milk, for the treatment of white matter injury (WMI) in preterm infants to preclude CP.
TT-20 is a small molecule that reverses WMI and motor abnormalities in preclinical animal models. Unlike current palliative care options, TT-20 is a first-in-class remyelination treatment that can preclude CP and reduce lifetime costs.
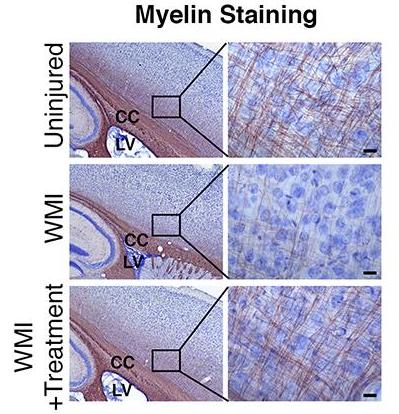
WMI results from the loss of preoligodendrocytes subsequent to inflammation or hypoxic injury experienced by premature infants in the NICU. The loss of these myelinating precursors results in significant and permanent hypomyelination and associated clinical deficits.
Dr. Benner and his team have identified multiple oxysterols in human maternal breast milk that promote the production of new oligodendrocytes from postnatal neural stem cells. In a mouse model of neonatal inflammatory WMI, administration of TT-20 promoted the production of new periventricular oligodendrocytes, improved myelination in the white matter regions most commonly injured in premature babies and rescued associated motor deficits.
Significance
Diffuse white matter injury is the most common brain injury leading to poor neurologic outcomes in survivors of premature birth. The clinical outcomes are diverse depending on the severity and its precise location but may include motor disabilities such as cerebral palsy (CP), which is the most common motor disability in childhood.
Population-based studies have estimated that ~3 per 1,000 children in the United States are affected and this number is higher in developing countries. The greatest risk factor for the development of CP is premature birth, with an inverse relationship between CP risk and gestational age and birth weight.
Per the CDC, CP has been reported in up to 10% of survivors of premature birth. The economic impact of this injury is high with lifetime costs of care for one child with CP estimated at 1.5 million U.S. dollars. Other outcomes of neonatal WMI include permanent cognitive and/or neurosensory impairment.
There are no treatment options for infants with this injury, only supportive care. Therefore, the development of an effective therapy would constitute a major medical breakthrough in this underserved patient population.
Benefits
Demonstrated benefits are those that have been observed and are verifiable.
Potential benefits are those logically expected with moderate to high confidence.
Use of oxysterols to prevent Cerebral Palsy (CP). (Demonstrated in animal models.) demonstrated.
Clinical
Prevention of CP will increase life expectancy for infants with WMI, improve quality of life of patients, and reduce caregiver burden. potential.
Community
Worldwide license for use of oxysterols for repair of myelin. demonstrated.
Economic
Launch of a startup (Tellus Therapeutics) to develop TT-20. demonstrated.
Economic
Two patents filed for use of oxysterols for myelin repair and treatment of inflammation-related diseases. demonstrated.
Economic
Reduced lifetime costs associated with CP, resulting in lower societal and financial cost of illness. potential.
Economic
This research has clinical, community, and economic implications. The framework for these implications was derived from the Translational Science Benefits Model created by the Institute of Clinical & Translational Sciences at Washington University in St. Louis.
Clinical
Dr. Benner and his team are developing an intravenous formulation of a drug to treat WMI in preterm infants, to preclude CP.
Currently, there is no treatment for WMI in preterm infants, and no treatment or prevention for CP. As of April 2020, pilot data have been collected in rodent models, and early IND-enabling studies are on-track with the aim of submitting a pre-IND meeting request to the FDA in Q3 2021.
The team plans to file an IND and initiate a phase 1b clinical trial as early as Q4 2022 to obtain initial safety data and determine optimal drug dosing for this population. The end result will be an intravenous formulation for preterm infants that is a first-in-class remyelination treatment able to prevent CP and reduce lifetime costs.
Community
One of the benefits of this technology is the potential for preventing CP, which will greatly improve the quality of life for patients and those around them, while significantly reducing lifetime care costs and the associated caregivers’ burden.
Prevention of CP or the reduction of severity will be a lifelong benefit for affected infants and their communities.
Economic
Shorter-term economic benefits include license agreements and patents to protect the intellectual property of the use of TT-20. A startup company, Tellus Therapeutics Inc., was established in October 2018 and is leading the efforts to translate the technology for clinical use in the pediatric population.
Longer-term economic benefits include cost savings and effectiveness, including the
potential to avoid the approximately $1.5 million U.S. lifetime costs for caring for one child with CP, decreasing caregiver burden, and improving quality of life of patients.
This technology doesn’t just have the capacity to lower healthcare costs, however: this technology is designed to save lives and improve the quality of life of patients.